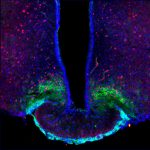
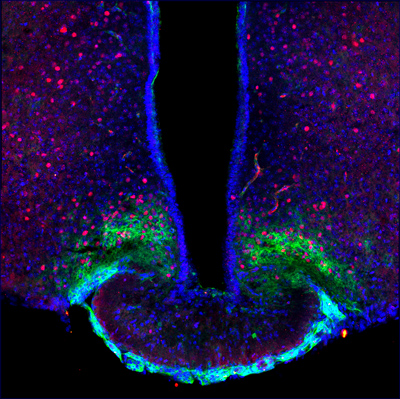
A plastic aggrecan barrier modulated by peripheral energy state gates metabolic signal access to arcuate neurons
Nat. Commun. 2024 Aug 7;15(1):6701.
Kuczynski-Noyau L, Karmann S, Alberton P, Martinez-Corral I, Nampoothiri S, Sauvé F, Lhomme T,
Apte SS, Bouret S, Aszodi A, Rasika S, Dam J, Prévot V and Mattot V.
MAGP-1 and fibronectin control EGFL7 functions by driving its deposition into distinct endothelial extracellular matrix locations.
FEBS J. 2018 Dec;285(23):4394-4412.
Villain G, Lelievre E, Broekelmann T, Gayet O, Havet C, Werkmeister E, Mecham R, Dusetti N, Soncin F, Mattot V.
miR-126-5p promotes retinal endothelial cell survival through SetD5 regulation in neurons.
Development. 2018 Jan 8;145(1).
Villain G, Poissonnier L, Noueihed B, Bonfils G, Rivera JC, Chemtob S, Soncin F, and Mattot V.
Endothelial Cell Activation Is Regulated by Epidermal Growth Factor−like Domain 7 (Egfl7) during Inflammation.
J Biol Chem. 2016 Nov 11;291(46):24017−24028.
Pinte S, Caetano B, Le Bras A, Havet C, Villain G, Dernayka R, Duez C, Mattot V, Soncin F.
miR126-5p repression of ALCAM and SetD5 in endothelial cells regulates leukocyte adhesion and transmigration.
Cardiovasc Res. 2014 Jun 1;102(3):436-47.
Poissonnier L, Villain G, Soncin F, Mattot V.
Egfl7 promotes tumor escape from immunity by repressing endothelial cell activation.
Cancer Res. 2011 Dec 1;71(23):7176-86.
Delfortrie S, Pinte S, Mattot V, Samson C, Villain G, Caetano B, Lauridant-Philippin G, Baranzelli MC, Bonneterre J, Trottein F, Faveeuw C, Soncin F.
VE-statin/egfl7 regulates vascular elastogenesis by interacting with lysyl oxidases.
EMBO J. 2008 ; 27 : 1658-70.
Lelièvre E, Hinek A, Lupu F, Buquet C, Soncin F, Mattot V.
VE-statin, an endothelial repressor of smooth muscle cell migration.
EMBO J. 2003 ; 22 : 5700-11.
Soncin F, Mattot V, Lionneton F, Spruyt N, Lepretre F, Begue A, Stehelin D.
Loss of the VEGF(164) and VEGF(188) isoforms impairs postnatal glomerular angiogenesis and renal arteriogenesis in mice.
J Am Soc Nephrol. 2002 ; 13 :1548-60.
Mattot V, Moons L, Lupu F, Chernavvsky D, Gomez RA, Collen D, Carmeliet P.
Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis.
Cell. 1999 ; 98 :147-57.
Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oostuyse B, Dewerchin M, Zanetti A, Angellilo A, Mattot V, Nuyens D, Lutgens E, Clotman F, de Ruiter MC, Gittenberger-de Groot A, Poelmann R, Lupu F, Herbert JM, Collen D, Dejana E.